Pine cone shell-based activated carbon used for CO2 adsorption†
Abstract
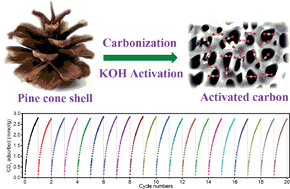
In this study, pine cone shell-based activated carbons were used to adsorb CO2. After a carbonization process at 500 °C, the resulting preliminary activated carbons (Non-PAC) were activated under different conditions. The results indicated good CO2 adsorption performance of pine cone shell-based activated carbons. For example, after activation at 650 °C and with a KOH : Non-PAC ratio of 2, the activated carbon (named as PAC-650/2) achieved a high CO2 adsorption capacity of 7.63 mmol g−1 and 2.35 mmol g−1 at 0 °C under 1 and 0.15 bar pressure, respectively. To determine the potential correlation between the amount of CO2 adsorbed and micropore distribution, linear correlations between cumulative pore volume over different ranges and amount of CO2 adsorbed were analyzed. Results showed that pores <0.70 nm played an important role in the CO2 adsorption process at 0 °C and 0.1 bar, and in contrast to previous research, pore volumes <0.80 nm or 0.82 nm did not show good linear correlation with the amount of CO2 adsorbed at 0 °C and 1 bar, and we inferred that this was most likely due to the unique pore structure of pine cone shell-based activated carbons. The highest Brunauer–Emmett–Teller (BET) surface area of 3931 m2 g−1 was obtained after activation at 800 °C and with a KOH : Non-PAC ratio of 2, but the highest BET surface area did not result in the highest CO2 adsorption capacity. This is mainly due to the BET surface area having regions unavailable for CO2 adsorption. X-ray photoelectron spectroscopy (XPS) analysis results for all activated carbons indicated a higher stability of pyridonic-N than pyridinic-N. Furthermore, in order to better understand the interaction between CO2 and pine cone shell-based activated carbons, we analyzed the isosteric heat of adsorption (Qst). Qst was higher than 22 kJ mol−1 for all activated carbons, and the highest initial isosteric heat of adsorption of 32.9 kJ mol−1 was obtained for the carbon activated at 500 °C and a KOH : Non-PAC ratio of 1. The optimal Qst (Qst,opt) under the conditions of a vacuum swing adsorption (at 25 °C, adsorption under 1 bar and desorption under 0.1 bar) process was 30 kJ mol−1.

 Please wait while we load your content...
Please wait while we load your content...