Lithium-ion conductivity in Li6Y(BO3)3: a thermally and electrochemically robust solid electrolyte†
Abstract
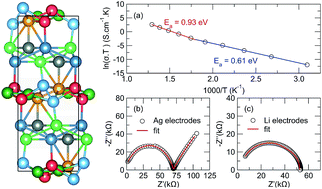
The development of new frameworks for solid electrolytes exhibiting fast Li-ion diffusion is critical for enabling new energy storage technologies. Here, we present a combined experimental and computational investigation into the ionic conductivity of Li6Y(BO3)3, a new class of solid electrolytes with a pseudo-layered structure. Temperature-dependent impedance spectroscopy shows the pristine material exhibits an ionic conductivity of 2.2 × 10−3 S cm−1 around 400 °C, despite the fact that density functional theory calculations point to multiple remarkably low-energy diffusion pathways. Our calculations indicate small energy barriers for lithium interstitials to diffuse along one-dimensional channels oriented in the c-direction, and also for lithium vacancies diffusing within ac planes. This coexistence of diffusion mechanisms indicates that Li6Y(BO3)3 is an extremely versatile host for exploring and understanding mechanisms for lithium-ion conductivity. We also find no evidence for reactivity with moisture in the atmosphere and that the material appears electrochemically stable when in direct contact with metallic lithium. This robust stability, alongside ionic conductivity that can be manipulated through appropriate aliovalent substitution, make Li6Y(BO3)3 an exceptionally promising new class of solid electrolyte.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials


 Please wait while we load your content...
Please wait while we load your content...