Pt–MoO3–RGO ternary hybrid hollow nanorod arrays as high-performance catalysts for methanol electrooxidation†
Abstract
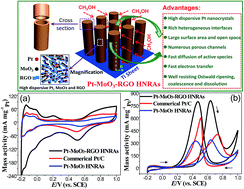
Here we design and synthesize novel Pt–MoO3–RGO (reduced graphene oxide) ternary hybrid hollow nanorod arrays (HNRAs) as anode catalysts for methanol electrooxidation. These fabricated Pt–MoO3–RGO HNRAs have highly dispersive MoO3, RGO, and Pt nanocrystals (∼3 nm), which leads to rich heterogeneous interfaces and strong synergistic effects among Pt, MoO3 and RGO. The Pt–MoO3–RGO HNRAs exhibit a high electrochemically active surface area (ECSA) of 71.20 m2 per (g, Pt), which is much higher than those of Pt–MoO3 HNRAs (34.23 m2 per (g, Pt)) and commercial Pt/C catalysts (52.89 m2 per (g, Pt)). Because of the strong synergistic effects and structural advantages, these Pt–MoO3–RGO HNRAs show much enhanced electrocatalytic activity, durability and CO anti-poisoning ability compared with Pt–MoO3 HNRAs and commercial Pt/C catalysts. Besides, the electrocatalytic activity of Pt–MoO3–RGO HNRAs also exceeds those of many Pt-based catalysts reported in the literature. Our finding demonstrates the importance of the interfacial and structural effects in harnessing the true electrocatalytic potential of Pt-based catalysts and will open up new strategies for the development of high-performance catalysts for methanol electrooxidation.

 Please wait while we load your content...
Please wait while we load your content...