Cell-sized liposome doublets reveal active tension build-up driven by acto-myosin dynamics†
Abstract
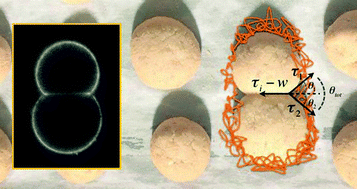
Cells modulate their shape to fulfill specific functions, mediated by the cell cortex, a thin actin shell bound to the plasma membrane. Myosin motor activity, together with actin dynamics, contributes to cortical tension. Here, we examine the individual contributions of actin polymerization and myosin activity to tension increase with a non-invasive method. Cell-sized liposome doublets are covered with either a stabilized actin cortex of preformed actin filaments, or a dynamic branched actin network polymerizing at the membrane. The addition of myosin II minifilaments in both cases triggers a change in doublet shape that is unambiguously related to a tension increase. Preformed actin filaments allow us to evaluate the effect of myosin alone while, with dynamic actin cortices, we examine the synergy of actin polymerization and myosin motors in driving shape changes. Our assay paves the way for a quantification of tension changes triggered by various actin-associated proteins in a cell-sized system.

 Please wait while we load your content...
Please wait while we load your content...