Kinetics of liquid–liquid phase separation in protein solutions exhibiting LCST phase behavior studied by time-resolved USAXS and VSANS†
Abstract
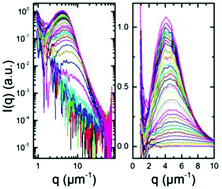
We study the kinetics of the liquid–liquid phase separation (LLPS) and its arrest in protein solutions exhibiting a lower critical solution temperature (LCST) phase behavior using the combination of ultra-small angle X-ray scattering (USAXS) and very-small angle neutron scattering (VSANS). We employ a previously established model system consisting of bovine serum albumin (BSA) solutions with YCl3. We follow the phase transition from sub-second to 104 s upon an off-critical temperature jump. After a temperature jump, the USAXS profiles exhibit a peak that grows in intensity and shifts to lower q values with time. Below 45 °C, the characteristic length scale (ξ) obtained from this scattering peak increases with time with a power of about 1/3 for different sample compositions. This is in good agreement with the theoretical prediction for the intermediate stage of spinodal decomposition where the growth is driven by interface tension. Above 45 °C, ξ follows initially the 1/3 power law growth, then undergoes a significant slowdown, and an arrested state is reached below the denaturation temperature of the protein. This growth kinetics may indicate that the final composition of the protein-rich phase is located close to the high density branch of the LLPS binodal when a kinetically arrested state is reached.


 Please wait while we load your content...
Please wait while we load your content...