Opposite counter-ion effects on condensed bundles of highly charged supramolecular nanotubes in water†
Abstract
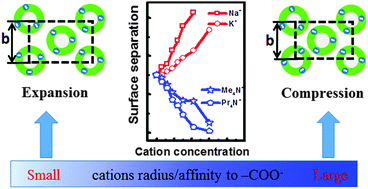
Although ion specificity in aqueous solutions is well known, its manifestation in unconventional strong electrostatic interactions remains implicit. Herein, the ionic effects in dense packing of highly charged polyelectrolytes are investigated in supramolecular nanotube prototypes. Distinctive behaviors of the orthorhombic arrays composed of supramolecular nanotubes in various aqueous solutions were observed by Small Angle X-ray Scattering (SAXS), depending on the counter-ions' size and affiliation to the surface –COO− groups. Bigger tetra-alkyl ammonium (TAA+) cations weakly bonding to –COO− will compress the orthorhombic arrays, while expansion is induced by smaller alkaline metal (M+) ions with strong affiliation to –COO−. Careful analysis of the changes in the SAXS peaks with different counter/co-ion combinations indicates dissimilar mechanisms underlying the two explicit types of ionic effects. The pH measurements are in line with the ion specificity by SAXS and reveal the strong electrostatic character of the system. It is proposed that the small distances between the charged surfaces, in addition to the selective adsorption of counter-ions by the surface charge, bring out the observed distinctive ionic effects. Our results manifest the diverse mechanisms and critical roles of counter-ion effects in strong electrostatic interactions.

 Please wait while we load your content...
Please wait while we load your content...