Synergistic effect of pH-responsive wormlike micelles based on a simple amphiphile†
Abstract
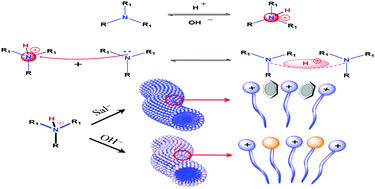
Imagine a novel solution that can be switched reversibly from low viscosity to high viscosity with only one additive, upon different commands. To this end, we have developed a simple and effective route to form smart, multi-response wormlike micelles based on a synthesized surfactant, N-cetyl-N,N-diisopropanolammonium bromide (CDIAB). Moreover, we provide new insight into the effects of synergy on this smart wormlike micelle. Rheological measurements were used to study the morphology of the wormlike micelles; 1H NMR spectroscopy and density functional theory (DFT) calculations were employed to investigate the molecular arrangements and mechanism of the synergy involved in the reversible reactions of pH-response and CO2-response of the micelles in solution. Based on the abovementioned results, it is encouraging to discover that binding energy and electrostatic interaction are the basic driving forces in the formation of wormlike micelles. Moreover, stable viscoelastic behavior was observed in the CDIAB system, with strong binding energy and electrostatic interactions. It is highly anticipated that the synergy observed in this surfactant will be of particular interest due to its novel mechanism and unique properties.

 Please wait while we load your content...
Please wait while we load your content...