Cellular uptake and intracellular fate of protein releasing bacterial amyloids in mammalian cells†
Abstract
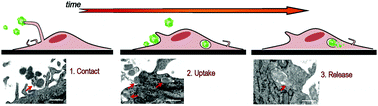
Bacterial Inclusion Bodies (IBs) are amyloidal protein deposits that functionally mimic secretory granules from the endocrine system. When formed by therapeutically relevant proteins, they complement missing intracellular activities in jeopardized cell cultures, offering an intriguing platform for protein drug delivery in substitutive therapies. Despite the therapeutic potential of IBs, their capability to interact with eukaryotic cells, cross the cell membrane and release their functional building blocks into the cytosolic space remains essentially unexplored. We have systematically dissected the process by which bacterial amyloids interact with mammalian cells. An early and tight cell membrane anchorage of IBs is followed by cellular uptake of single or grouped IBs of variable sizes by macropinocytosis. Although an important fraction of the penetrating particles is led to lysosomal degradation, biologically significant amounts of protein are released into the cytosol. In addition, our data suggest the involvement of the bacterial cell folding modulator DnaK in the release of functional proteins from these amyloidal reservoirs. The mechanisms supporting the internalization of disintegrable protein nanoparticles revealed here offer clues to implement novel approaches for protein drug delivery based on controlled protein packaging as bacterial IBs.


 Please wait while we load your content...
Please wait while we load your content...