Segregation of “isotope” particles within colloidal molecules†
Abstract
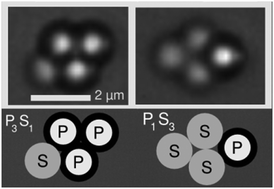
Clusters of spherical particles are called “colloidal molecules” because they adopt structures that resemble those of true molecules. In this analogy, the particles are the atoms, the attractive interactions between them are bonds, and the different structures that appear in equilibrium are isomers. We take this analogy a step further by doping colloidal molecules with colloidal “isotopes,” particles that have the same size but different bonding energies from the other particles in the system. Our molecules are two-dimensional clusters consisting of polystyrene and silica microspheres held together by depletion interactions. Using a combination of optical microscopy and particle tracking, we examine an ensemble of 4- and 5-particle molecules at different isotope ratios. We find that the isotopes tend to segregate to particular positions in the various isomers. We explain these findings using a statistical mechanical model that accounts for the rotational entropy of the isomers and the different interaction potentials between the different types of particles. The model shows how to optimize the yield of any particular isomer, so as to put the isotopes in desired locations. Our experiments and models show that even in systems of particles with isotropic interactions, the structures of self-assembled molecules can in principle be controlled to a surprisingly high extent.

 Please wait while we load your content...
Please wait while we load your content...