Supramolecular polymerisation in water; elucidating the role of hydrophobic and hydrogen-bond interactions†
Abstract
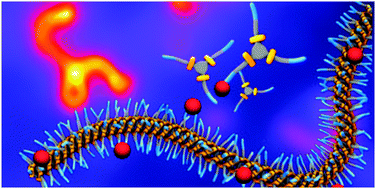
Understanding the self-assembly of small molecules in water is crucial for the development of responsive, biocompatible soft materials. Here, a family of benzene-1,3,5-tricarboxamide (BTA) derivatives that comprise a BTA moiety connected to an amphiphilic chain is synthesised with the aim to elucidate the role of hydrophobic and hydrogen-bonding interactions in the self-assembly of these BTAs. The amphiphilic chain consists of an alkyl chain with a length of 10, 11, or 12 methylene units, connected to a tetraethylene glycol (at the periphery). The results show that an undecyl spacer is the minimum length required for these BTAs to self-assemble into supramolecular polymers. Interestingly, exchange studies reveal only minor differences in exchange rates between BTAs containing undecyl or dodecyl spacers. Additionally, IR spectroscopy provides the first experimental evidence that hydrogen-bonding is operative and contributes to the stabilisation of the supramolecular polymers in water.
- This article is part of the themed collection: Open access articles from Soft Matter
 Please wait while we load your content...
Please wait while we load your content...