Steric stabilization of nanoparticles with grafted low molecular weight ligands in highly concentrated brines including divalent ions†
Abstract
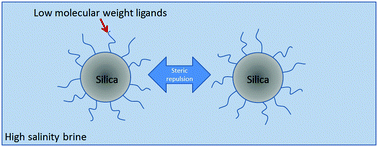
Whereas numerous studies of stabilization of nanoparticles (NPs) in electrolytes have examined biological fluids, the interest has grown recently in media with much higher ionic strengths including seawater and brines relevant to environmental science and subsurface oil and gas reservoirs. Given that electrostatic repulsion is limited at extremely high ionic strengths due to charge screening, we have identified ligands that are well solvated in concentrated brine containing divalent cations and thus provide steric stabilization of silica nanoparticles. Specifically, the hydrodynamic diameter of silica nanoparticles with grafted low molecular weight ligands, a diol ether, [3-(2,3-dihydroxypropoxy)propyl]-trimethoxysilane, and a zwitterionic sulfobetaine, 3-([dimethyl(3-trimethoxysilyl)propyl]ammonio)propane-1-sulfonate, is shown with dynamic light scattering to remain essentially constant, indicating lack of aggregation, at room temperature and up to 80 °C for over 30 days. An extended DLVO model signifies that steric stabilization is strongly dominant against van der Waals attraction for ∼10 nm particles given that these ligands are well solvated even in highly concentrated brine. In contrast, polyethylene glycol oligomers do not provide steric stabilization at elevated temperatures, even at conditions where the ligands are soluble, indicating complicating factors including bridging of the ether oxygens by divalent cations.

 Please wait while we load your content...
Please wait while we load your content...