A colour-tunable chiral AIEgen: reversible coordination, enantiomer discrimination and morphology visualization†
Abstract
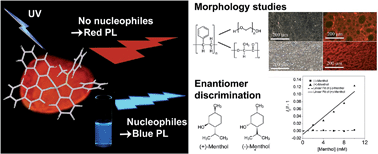
We present a conceptually new approach to synthesise a boron-containing Aggregation-Induced Emissive Luminogen (AIEgen) with a chiral chromophore. An intramolecular N–B coordinating bond results in a low-energy transition that renders the material red-emissive in a solid state. By competitive binding of nucleophiles to the boron atom, this bond is replaced in favour of an intermolecular coordinating bond, which results in a tremendous blue-shift in both the absorption and emission. A supportive DFT computation elucidates that a breakage of the intramolecular N–B coordinating bond causes a tremendous loss of conjugation in the LUMO, resulting in a larger energy gap. Owing to the fact that our scaffold is intrinsically chiral and Lewis-acidic, we demonstrate how our AIEgen discriminates between two pairs of enantiomers in a simple UV-vis measurement. Furthermore, the binding capabilities are exploited to stain polymer blends that comprised a non-coordinating and a Lewis-basic polymer. The red fluorescence that originates only from domains of the non-coordinating polymer is conveniently detected by a fluorescence microscope. Thus, compared to current analytical methods, we present a cheaper and faster methodology to study the micro-morphologies of certain polymer blends.

 Please wait while we load your content...
Please wait while we load your content...