Bimodal supramolecular functionalization of carbon nanotubes triggered by covalent bond formation†
Abstract
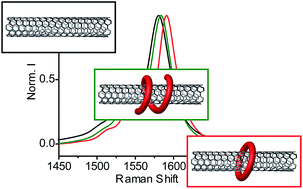
Many applications of carbon nanotubes require their chemical functionalization. Both covalent and supramolecular approaches have been extensively investigated. A less trodden path is the combination of both covalent and noncovalent chemistries, where the formation of covalent bonds triggers a particularly stable noncovalent interaction with the nanotubes. We describe a series of naphthalene diimide (NDI) bisalkene molecules that, upon mixing with single-walled carbon nanotubes (SWNTs) and Grubbs' catalyst, undergo two different reaction pathways. On one hand, they ring-close around the SWNTs to form rotaxane-like mechanically interlocked derivatives of SWNTs (MINTs). Alternatively, they oligomerize and then wrap around the SWNTs. The balance of MINTs to oligomer-wrapped SWNTs depends on the affinity of the NDI molecules for the SWNTs and the kinetics of the metathesis reactions, which can be controlled by varying the solvent. Thorough characterization of the products (TGA, TEM, AFM, Raman, UV-vis-NIR, PLE, XPS and UPS) confirms their structure and shows that each type of functionalization affects the electronic properties of the SWNTs differently.


 Please wait while we load your content...
Please wait while we load your content...