Atmospheric-pressure ionization and fragmentation of peptides by solution-cathode glow discharge†
Abstract
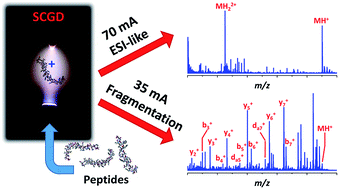
Modern “-omics” (e.g., proteomics, glycomics, metabolomics, etc.) analyses rely heavily on electrospray ionization and tandem mass spectrometry to determine the structural identity of target species. Unfortunately, these methods are limited to specialized mass spectrometry instrumentation. Here, a novel approach is described that enables ionization and controlled, tunable fragmentation of peptides at atmospheric pressure. In the new source, a direct-current plasma is sustained between a tapered metal rod and a flowing sample-containing solution. As the liquid stream contacts the electrical discharge, peptides from the solution are volatilized, ionized, and fragmented. At high discharge currents (e.g., 70 mA), electrospray-like spectra are observed, dominated by singly and doubly protonated molecular ions. At lower currents (35 mA), many peptides exhibit extensive fragmentation, with a-, b-, c-, x-, and y-type ion series present as well as complex fragments, such as d-type ions, not previously observed with atmospheric-pressure dissociation. Though the mechanism of fragmentation is currently unclear, observations indicate it could result from the interaction of peptides with gas-phase radicals or ultraviolet radiation generated within the plasma.

 Please wait while we load your content...
Please wait while we load your content...