Carbonyl-assisted reverse regioselective cascade annulation of 2-acetylenic ketones triggered by Ru-catalyzed C–H activation†
Abstract
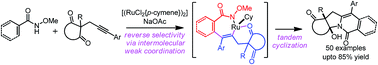
The first reverse regioselective intermolecular annulation of aryl substituted 2-acetylenic ketones with O-substituted N-hydroxybenzamides or acrylamides followed by tandem cyclization via ruthenium-catalyzed C–H activation, is reported. Excellent reverse selectivity of alkyne insertion was induced by the weak coordination between the carbonyl group and ruthenium complex. This highly efficient and practical reaction has a broad range of substrate scope with excellent functional-group tolerance. The tandem reaction provides a wide range of polycyclic products that have an indozilidine structural motif, and are found to potentially be synthetically and pharmaceutically valuable.

 Please wait while we load your content...
Please wait while we load your content...