Metal-free disproportionation of formic acid mediated by organoboranes†
Abstract
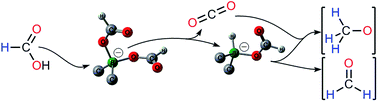
In the presence of dialkylboranes, formic acid can be converted to formaldehyde and methanol derivatives without the need for an external reductant. This reactivity, in which formates serve as the sole carbon and hydride sources, represents the first example of the disproportionation of formate anions under metal-free conditions. Capitalizing on both experimental and computational (DFT) mechanistic considerations, the role of transient borohydride is highlighted in the reduction of formates and this reactivity was further exemplified in the methylation of TMP (2,2,6,6-tetramethylpiperidine) and in the transfer hydroboration reactions for the reduction of aldehydes.

 Please wait while we load your content...
Please wait while we load your content...