Visible-light-driven CO2 reduction on a hybrid photocatalyst consisting of a Ru(ii) binuclear complex and a Ag-loaded TaON in aqueous solutions†
Abstract
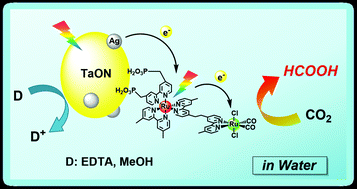
A hybrid photocatalyst consisting of a Ru(II) binuclear complex and a Ag-loaded TaON reduced CO2 by visible light even in aqueous solution. The distribution of the reduction products was strongly affected by the pH of the reaction solution. HCOOH was selectively produced in neutral conditions, whereas the formation of HCOOH competed with H2 evolution in acidic conditions. Detailed mechanistic studies revealed that the photocatalytic CO2 reduction proceeded via ‘Z-schematic’ electron transfer with step-by-step photoexcitation of TaON and the photosensitizer unit in the Ru(II) binuclear complex. The maximum turnover number for HCOOH formation was 750 based on the Ru(II) binuclear complex under visible-light irradiation, and the optimum external quantum efficiency of the HCOOH formation was 0.48% using 400 nm monochromic light with ethylenediaminetetraacetic acid disodium salt as a sacrificial reductant. Even in aqueous solution, the hybrid could also convert visible-light energy into chemical energy (ΔG0 = +83 kJ mol−1) by the reduction of CO2 to HCOOH with methanol oxidation.
- This article is part of the themed collections: In celebration of Kazunari Domen’s 65th birthday, 2018 and Global Energy Challenges: Hydrogen Energy

 Please wait while we load your content...
Please wait while we load your content...