Dynamic covalent synthesis of aryleneethynylene cages through alkyne metathesis: dimer, tetramer, or interlocked complex?†
Abstract
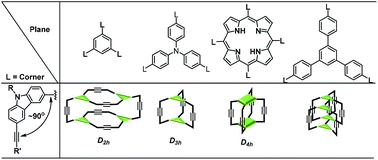
A dynamic covalent approach towards rigid aryleneethynylene covalent organic polyhedrons (COPs) was explored. Our study on the relationship of the COP structures and the geometry of their building blocks reveals that the topology of aryleneethynylene COPs strongly depends on the size of the building blocks. A tetramer (D2h symmetric), dimer, or interlocked complex can be formed from monomers with the same face-to-edge angle but in different sizes. As alkyne metathesis is a self-exchange reaction and non-directional, the cyclooligomerization of multi-alkyne monomers involves both intramolecular cyclization and intermolecular metathesis reaction, resulting in complicated thermodynamic process disturbed by kinetic competition. Although a tetrahedron-shaped tetramer (Td symmetric) has comparable thermodynamic stability to a D2h symmetric tetramer, its formation is kinetically disfavored and was not observed experimentally. Aryleneethynylene COPs consist of purely unsaturated carbon backbones and exhibit large internal cavities, which would have interesting applications in host–guest chemistry and development of porous materials.


 Please wait while we load your content...
Please wait while we load your content...