Electronic structure and reactivity of nickel(i) pincer complexes: their aerobic transformation to peroxo species and site selective C–H oxygenation†
Abstract
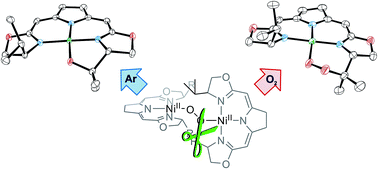
The study is aimed at a deeper understanding of the electronic structure of the T-shaped nickel(I) complex [LigiPr(iso)Ni] (1b), bearing the iso-PyrrMeBox (bis(oxazolinylmethylidene)pyrrolidinido) pincer ligand, and its CO adduct [LigiPr(iso)Ni(CO)] (2b) as well as to provide insight into the mechanism of autoxidation of the different nickel peroxo species of this ligand type. CO was found to react reversibly with complex 1b resulting in the corresponding CO adduct 2b. The EPR data as well as the results of DFT modeling revealed significant differences in the electronic structure of 1b and 2b. Reaction of [LigPh(iso)Ni] and [LigiPr(iso)Ni] (1a and b) with dioxygen yielded the 1,2-μ-peroxo complexes [Lig(iso)NiO]23a and b which reacted with hydrogen peroxide to give the hydroperoxo complexes [Lig(iso)NiOOH] 5a and b. Thermal aerobic decomposition of the peroxo species 3a and 5a in the presence of O2 led to a C–H activation of the ligand at the benzylic position of the oxazoline ring forming diastereomeric cyclic peroxo complexes 6 and 6′. For the 1,2-μ-peroxo complex 3b the autoxidation of the pincer in the absence of O2 occurred at the tertiary C–H bond of the iPr-group and led to a selective formation of the terminal hydroxo complex [LigiPr(iso)NiOH] 7b and the cyclic alkoxy complex 8 in equimolar quantities, while the corresponding cyclic peroxo species 9 was formed along with 7b in the presence of oxygen. Whether or not O–O bond cleavage occurred in the generation of 9 was established upon performing labeling experiments which indicate that the transformation does not involve an initial O–O bond cleaving step. Based on these observations and a series of stoichiometric transformations a tentative proposal for the processes involved in the anaerobic and aerobic decomposition of 3b has been put forward. Finally, the nickel(II) methyl complex [LigPh(iso)NiMe] 14 reacted with O2 to give the methylperoxo complex [LigPh(iso)NiOOMe] 15 which slowly converted to a mixture of near equal amounts of the formato and the hydroxo complexes, [LigPh(iso)NiOOCH] 16 and [LigPh(iso)NiOH] 7a, along with half an equivalent of methanol. The formato complex 16 itself decomposed at elevated temperatures to CO2, dihydrogen as well as the nickel(I) species 1a.

 Please wait while we load your content...
Please wait while we load your content...