Isolation of Au-, Co-η1PCO and Cu-η2PCO complexes, conversion of an Ir–η1PCO complex into a dimetalladiphosphene, and an interaction-free PCO anion†
Abstract
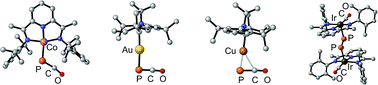
Sodium phosphaethynolate reacts with [MCl(PDI)] (M = Co, Ir; PDI = pyridinediimine) to give metallaphosphaketenes, which in the case of iridium rearranges into a dimetalladiphosphene, via CO migration from phosphorus to the metal. Two different bonding modes of the PCO anion to CAAC-coinage metal complexes [CAAC: cyclic (alkyl)(amino)(carbene)] are reported, one featuring a strong Au–P bond and the other an η2 coordination to copper. The gold complex appears to be mostly unreactive whereas the copper complex readily reacts with various organic substrates. A completely free PCO anion was structurally characterized as the [Cu(La)2]+ (OCP)− salt. It results from the simple displacement of the PCO unit of the cationic (CAAC)Cu(PCO) complex by a second equivalent of CAAC.

 Please wait while we load your content...
Please wait while we load your content...