Chemo- and regioselective oxygenation of C(sp3)–H bonds in aliphatic alcohols using a covalently bound directing activator and atmospheric oxygen†
Abstract
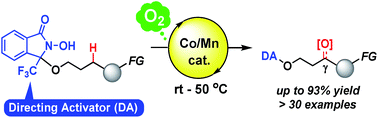
Chemically reactive directing groups (directing activators) represent a promising strategy for mild and regioselective C(sp3)–H functionalization. The use of a radical N-oxyl directing activator promoted the aerobic oxygenation of benzylic, propargylic, tertiary, and unactivated acyclic methylene C(sp3)–H bonds in aliphatic alcohols with γ- (or δ-) selectivity under mild conditions (room temperature to 50 °C). The reaction was unaffected by the presence of various oxidation-sensitive functional groups, which proved to be problematic in previously reported studies on the oxidation of C(sp3)–H bonds. Structural modifications on the directing activator altered the regioselectivity, and thus provided an ultra-remote aerobic C(sp3)–H oxygenation. The observed reactivity and regioselectivity could be rationalized in terms of the intramolecular conformational accessibility of the N-oxyl radical and the electronic characteristics of C(sp3)–H bonds.


 Please wait while we load your content...
Please wait while we load your content...