Palladium-catalyzed ligand-promoted site-selective cyanomethylation of unactivated C(sp3)–H bonds with acetonitrile†
Abstract
The direct cyanomethylation of unactivated sp3 C–H bonds of aliphatic amides was achieved via palladium catalysis assisted by a bidentate directing group with good functional group compatibility. This process represents the first example of the direct cross-coupling of sp3 C–H bonds with acetonitrile. Considering the importance of the cyano group in medicinal and synthetic organic chemistry, this reaction will find broad application in chemical research.
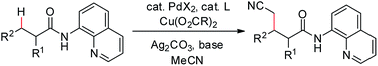

 Please wait while we load your content...
Please wait while we load your content...