Many Mg–Mg bonds form the core of the Mg16Cp *8Br4K cluster anion: the key to a reassessment of the Grignard reagent (GR) formation process?†
Abstract
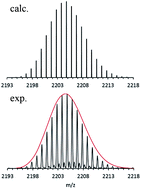
It caused a sensation eight years ago, when the first room temperature stable molecular compound with a Mg–Mg bond (LMgMgL, L = chelating ligand) containing magnesium in the oxidation state +1 was prepared. Here, we report the preparation of a [Mg16Cp*8Br4K]− cluster anion (Cp* = pentamethylcyclopentadiene) with 27 Mg–Mg bonds. It has been obtained through the reaction of KCp* with a metastable solution of MgBr in toluene. A highly-resolved Fourier transform mass spectrum (FT-MS) of this cluster anion, brought into vacuum by electrospraying its solution in THF, provides the title cluster's stoichiometry. This Mg16 cluster together with experiments on the metastable solution of MgBr show that: during the formation process of GRs (Grignard reagents) which are involved in most of sophisticated syntheses of organic products, not the highly reactive MgBr radical as often presumed, but instead the metalloid Mg16Cp*8Br4 cluster anion and its related cousins that are the operative intermediates along the pathway from Mg metal to GRs (e.g. Cp*MgBr).

 Please wait while we load your content...
Please wait while we load your content...