Accessing low-oxidation state taxanes: is taxadiene-4(5)-epoxide on the taxol biosynthetic pathway?†
Abstract
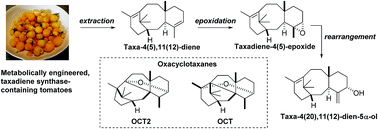
We have shown for the first time that taxadiene (3) can be epoxidised in a regio- and diastereoselective manner to provide taxadiene-4(5)-epoxide (12) as a single diastereoisomer, and that this epoxide can be rearranged to give taxa-4(20),11(12)-dien-5α-ol (4). Furthermore, the epoxide 12 rearranges under acidic conditions to give taxa-4(20),11(12)-dien-5α-ol (4), the known bridged ether OCT (5) and the new oxacyclotaxane (OCT2) 15. Contrary to previous speculation, taxadiene-4(5)-epoxide (12) is susceptible to rearrangement when exposed to an ironIII porphyrin, and these observations justify consideration of epoxide 12 as a chemically competent intermediate on the taxol biosynthetic pathway.


 Please wait while we load your content...
Please wait while we load your content...