Dual gold/photoredox-catalyzed C(sp)–H arylation of terminal alkynes with diazonium salts†
Abstract
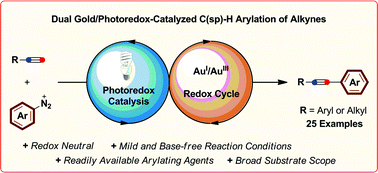
The arylation of alkyl and aromatic terminal alkynes by a dual gold/photoredox catalytic system is described. Using aryldiazonium salts as readily available aryl sources, a range of diversely-functionalized arylalkynes could be synthesized under mild, base-free reaction conditions using visible light from simple household sources or even sunlight. This process, which exhibits a broad scope and functional group tolerance, expands the range of transformations amenable to dual gold/photoredox catalysis to those involving C–H bond functionalization and demonstrates the potential of this concept to access AuI/AuIII redox chemistry under mild, redox-neutral conditions.
- This article is part of the themed collections: Top 50 Articles of 2016: Energy and Catalysis and Top 50 Articles of 2016: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...