Caffeine and nicotine in 3% NaCl solution with CO2 as corrosion inhibitors for low carbon steel†
Abstract
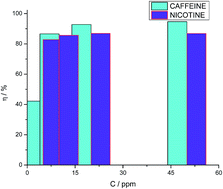
The electrochemical impedance technique was employed to evaluate the inhibitory activity of caffeine and nicotine with a concentration range from 0 to 50 ppm under static and turbulent flows (100, 500 and 1000 rpm) on the corrosion of AISI 1018 low-carbon steel immersed in NaCl + CO2. The inhibitor efficiency (η) increased with increasing inhibitor concentration, as corroborated by EIS. However, this effectiveness was even present at low inhibitor concentration, indicating that these compounds are good corrosion inhibitors under static conditions (η > 90% for caffeine and >80% for nicotine). Thermodynamics analysis using the Langmuir model demonstrated that the adsorption is by physisorption. Surface analysis (SEM-EDS) also corroborated that the corrosion inhibition occurs due to the adsorption of caffeine or nicotine molecules at the metal–solution surface. The influence of immersion time on η was also assessed for the best concentration, demonstrating that even when the η of both inhibitors diminished nearly linearly as a function of time, they provide adequate protection (η > 80%) to the steel substrate even after 336 hours of exposure to this highly corrosive medium, with the inhibition decreasing with further exposure.

 Please wait while we load your content...
Please wait while we load your content...