Synthesis and anti-tubercular activity of fused thieno-/furo-quinoline compounds†
Abstract
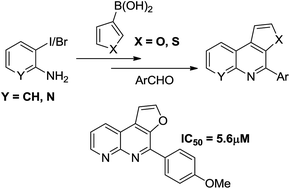
A simple route for preparation of 4-substituted thieno/furo[2,3-c]quinoline compounds is reported in this paper. These compounds were synthesized by using Suzuki coupling between appropriate boronic acid and 2-iodoaniline, followed by reaction with diverse aldehydes in the presence of a catalytic amount of FeCl3. Naphthyridine analogues were also prepared in order to demonstrate the efficacy of the developed method. Further anti-tubercular activity screening of the molecules was undertaken, wherein compounds bearing a fused furo[2,3-c][1,8]naphthyridine skeleton displayed highest activity. The best MIC (minimum inhibitory concentration) value of 5.6 μmol was obtained for 4-(4-methoxyphenyl)furo[2,3-c][1,8]naphthyridine, which is found to be superior to the existing first line anti-tubercular drug ethambutol (7.6 μmol).

 Please wait while we load your content...
Please wait while we load your content...