Approach to thiazole-containing tetrahydropyridines via Aza–Rauhut–Currier reaction and their potent fungicidal and insecticidal activity†
Abstract
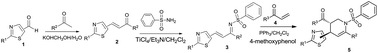
A convenient and efficient synthesis of multi-substituted thiazole-containing tetrahydropyridine moieties was reported using the phosphine-catalyzed Aza–Rauhut–Currier reaction with excellent yields and diastereoselectivity. Thiazole-containing tetrahydropyridines were further transformed into the corresponding piperidine derivatives. The biological activity of the title compounds was explored; they exhibited moderate insecticidal activity against Aphis laburni Kaltenbach at 100 μg mL. A 3D QSAR model accurately predicted the insecticidal activity of the structurally diverse set of test compounds. Thiazole-containing tetrahydropyridines were active against normal fungi and also had good activity against resistant fungi mutations without cross resistance; thus, these compounds will be valuable for resistance management. The predicted potential fungicidal target of the title compounds is fumarate reductase.

 Please wait while we load your content...
Please wait while we load your content...