Mechanistic studies on the VO(acac)2-catalyzed oxidative cleavage of lignin model compounds in acetic acid†
Abstract
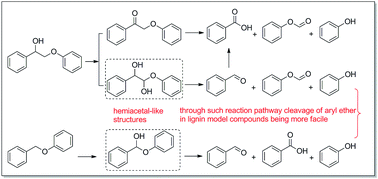
Lignin is the primary source of aromatic chemicals in nature. Selective aerobic oxidation has provided a promising approach for breaking lignin into smaller aromatics. During VO(acac)2-catalyzed oxidation of lignin model compound 2-phenoxy-1-phenylethanol, benzaldehyde was unexpectedly observed as a product, in contrast to the results reported previously. The reaction pathway was studied, and a hemiacetal-like intermediate was found to be involved, and the similar intermediate was also observed during oxidation of model compound benzyl phenyl ether. Compared with direct C–O bond cleavage, prior formation of hemiacetal-like structures via oxidation would be expected to facilitate the cleavage of the aryl ether bond in aralkyl aryl ethers under mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...