Multicomponent synthesis of hydrazino depsipeptides†
Abstract
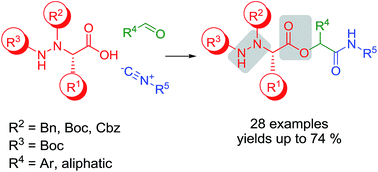
The Passerini reaction of α-hydrazino acids, carbonyl compounds and isocyanides yielded hydrazino depsipeptides, a new class of backbone extended peptidomimetics comprising amide bond isostere. A wide range of carbonyl and isocyano components were used along the α-hydrazino acids carrying three different Nα protecting groups. The kinetics and thermodynamic equilibrium of the rate-determining step of the reaction with different α-hydrazino acids were studied by DFT approach.

 Please wait while we load your content...
Please wait while we load your content...