Corrosion inhibition of X70 sheets by a film-forming imidazole derivative at acidic pH
Abstract
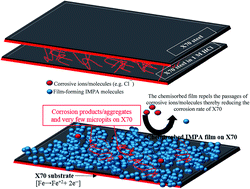
The anticorrosion potential of a chemisorbed film formed from 3-imidazol-1-ylpropan-1-amine (IMPA) against the degradation of X70 steel in 1 M HCl has been investigated using electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization techniques. IMPA has been found to reduce the corrosion of X70 in the aqueous medium at pH 0 to a great extent; an inhibition efficiency (IE%) up to 90% has been achieved for 500 ppm IMPA concentration at room temperature. Corrosion inhibition by IMPA is concentration-dependent and becomes more stable by virtue of adsorption of IMPA film on the X70 substrate. This adsorption phenomenon has been probed by scanning electron microscopy (SEM), atomic force microscopy (AFM) and infra-red (IR) spectroscopy. The formation of this film on the X70 surface fosters improved impedance against the flow of ionic currents of corrosive ions and molecules. IMPA acted as a mixed-type film-forming inhibitor as demonstrated from the results of potentiodynamic scans. The mechanism of X70 corrosion inhibition has been proposed using quantum structure/activity relations to explain the extent of influence of the molecular structure of IMPA on the corrosion inhibition of the IMPA film.


 Please wait while we load your content...
Please wait while we load your content...