Selective oxidative degradation of azo dyes by hydrogen peroxide catalysed by manganese(ii) ions†
Abstract
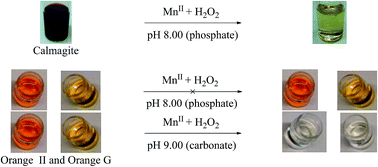
Manganese(II) ions catalyse the oxidative degradation of Calmagite (H3CAL) dye in aqueous solution at 20 ± 1 °C in the pH range 7.5–9.0 using hydrogen peroxide (H2O2) as oxidant by a mechanism that involves strong complexation to the MnII centre. It is proposed that [MnIII(CAL)(O2H)]− i.e. a dye coordinated hydroperoxyl (O2H−) MnIII complex is formed and bleaching of the dye is initiated by an electron-transfer to MnIII, with the binding of H2O2 being the rate determining step. At pH 9.0 in (bi)carbonate, HCO3−, H3CAL is rapidly bleached via the in situ formation of coordinated peroxycarbonate (HCO4−); a TOF (TOF = moles of dye bleached per mole of manganese per hour) of ∼5000 h−1 can be achieved. The bleaching of the related azo dyes Orange II and Orange G is different because, unlike Calmagite, they lack an o,o-dihydroxy motif so are unable to complex strongly to MnII and no oxidation to MnIII occurs. At pH 8.0 (phosphate buffer) Orange II and Orange G are not bleached but bleaching can be achieved at pH 9.0 (HCO3− buffer); the rate determining step is dye coordination and it is proposed bleaching is achieved via an outer-sphere oxygen atom transfer. Mechanisms for dye bleaching at pH 8.0 and pH 9.0 are proposed using data from EPR, UV/VIS and ESI-MS. MnII/H2O2/HCO3− form a potent oxidising mixture that is capable of removing stubborn stains such as curcumin.


 Please wait while we load your content...
Please wait while we load your content...