In situ formation of silk-gelatin hybrid hydrogels for affinity-based growth factor sequestration and release†
Abstract
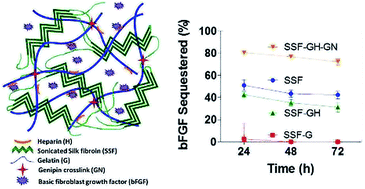
Silk fibroin (SF) and gelatin are natural polymers suitable for biomedical applications, including controlled protein release. SF offers high mechanical strength and slow enzymatic degradability, whereas gelatin contains bioactive motifs that can provide biomimicry to the resulting scaffolds. Owing to their complementary material properties, SF and gelatin are increasingly being used together to afford hybrid scaffolds with adjustable properties. Here, we report the use of in situ crosslinked SF/gelatin hydrogels as a platform for tunable growth factor sequestration and delivery. We demonstrate that the physical assembly of SF into insoluble networks could be accelerated by sonication even in the presence of gelatin. However, the processing conditions from which to prepare SF aqueous solution (e.g., heating duration and number of processing steps) drastically altered the resulting hydrogel physical properties. Furthermore, the stiffness of SF/gelatin hybrid gels displayed temperature dependency. Specifically, incorporation of gelatin increased gel stiffness at 25 °C but decreases hydrogel mechanical stability at 37 °C. The thermostability of SF/gelatin gels can be restored by using a low concentration of genipin, a naturally derived chemical crosslinker. We also incorporate heparin-conjugated gelatin (GH) into the hydrogels to create a hybrid matrix capable of sequestering growth factors, such as basic fibroblast growth factor (bFGF). Both sonicated SF (SSF) and hybrid SSF-GH gels exhibit moderate bFGF sequestration, but only SSF-GH gels afford slow release of bFGF. On the other hand, genipin-stabilized network exhibited the highest retention and sustained release of bFGF, suggesting the suitability of this particular formulation as a scaffold for tissue engineering applications.

 Please wait while we load your content...
Please wait while we load your content...