Use of ordered mesoporous silica-loaded phyto-phospholipid complex for BCS IV class plant drug to enhance oral bioavailability: a case report of tanshinone IIA
Abstract
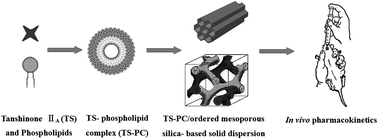
This work aimed to improve membrane permeability as well as in vitro dissolution rate and thus enhance oral bioavailability for a BCS IV class plant drug by constructing a combined drug delivery system (CDDS) composed of a phospholipid complex (PC) and an ordered mesoporous silica-based solid dispersion (SD), using tanshinone IIA (TS) as a model drug. Tanshinone IIA phospholipid complex (TS–PC) and ordered mesoporous silica-based solid dispersion (TSPC-SD) were prepared using the solvent evaporation method. Differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier transform infrared (FTIR) spectroscopy proved the formation of TS–PC or TSPC-SD, and amorphous form existing in these two systems. n-Octanol/water partition coefficient (lg Po/w) tests showed that a remarkably decreased lg Po/w for TS–PC was obtained compared to unformulated TS, while no increase was observed for TSPC-SD compared to TS–PC. Evaluations in Caco-2 cell monolayers revealed that TS–PC exhibited a significantly increased absorptive permeability (p < 0.05) in apparent permeability coefficients (Papp) (70.2% higher) compared to unformulated TS. Compared to that of unformulated TS, in vitro drug dissolution rate of TS from TSPC-SD significantly increased (p < 0.01), while that from TS–PC was slightly decreased. Pharmacokinetic studies demonstrated that TSPC-SD had 2.19-fold higher AUC0–t compared to unformulated TS (p < 0.01). The remarkable improvements in bioavailability with the use of TSPC-SD may result from comprehensive effects, including improved lg Po/w and Pappvia PC, and increased dissolution rates from SD. These results indicated that the CDDS can be promising for enhancing the oral bioavailability of BCS IV class drugs.

 Please wait while we load your content...
Please wait while we load your content...