An efficient one-pot catalyzed synthesis of 2,5-disubstituted-1,3,4-oxadiazoles and evaluation of their antimicrobial activities†
Abstract
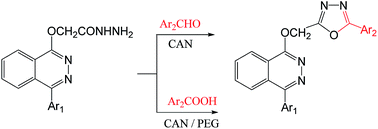
One-pot synthesis of 1,3,4-oxadiazole derivatives was achieved by treatment of 2-((4-(phenoxathiin-2-yl)phthalazin-1-yl)oxy)acetohydrazide with aromatic aldehydes in the presence of cerium(IV) ammonium nitrate in dichloromethane. This facile method was confirmed by the cyclization–oxidation reaction of the corresponding hydrazone by cerium(IV) ammonium nitrate to afford the same 1,3,4-oxadiazoles. Also, the reaction of 2-((4-(phenoxathiin-2-yl)phthalazin-1-yl)oxy)acetohydrazide with aromatic carboxylic acids in the presence of cerium(IV) ammonium nitrate in polyethylene glycol afforded 1,3,4-oxadiazole derivatives. The structural formulae of all products were confirmed and characterized by elemental analyses and spectral data. Most of the synthesized products were evaluated for their antibacterial and antifungal activities and showed potent to weak activity.

 Please wait while we load your content...
Please wait while we load your content...