Understanding the kinetics of thermal decomposition of 2,3-epoxy-2,3-dimethylbutane using RRKM theory†
Abstract
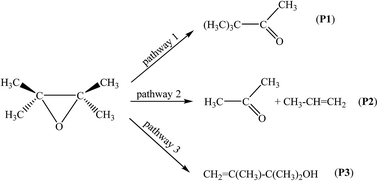
The thermal decomposition kinetics of 2,3-epoxy-2,3-dimethylbutane have been studied computationally using density functional theory, along with various exchange–correlation functionals and an extremely large basis set. The calculated energy profiles have been supplemented with calculations of kinetic rate constants and branching ratios under atmospheric pressure and in the fall-off regime have been supplied, using transition state theory (TST) and statistical Rice–Ramsperger–Kassel–Marcus (RRKM) theory. Kinetic rate constants and branching ratios under atmospheric pressure and in the fall-off regime have been supplied, using transition state and RRKM theories. By comparison with experiment, all our calculations indicate that, from a kinetic viewpoint, the most favorable process is thermal decomposition of 2,3-epoxy-2,3-dimethylbutane into the 2,3-dimethylbut-3-en-2-ol, whereas under thermodynamic control of the reactions, the most abundant product derived from the 2,3-epoxy-2,3-dimethylbutane species will be the 3,3-dimethylbutan-2-one species. The regioselectivity of the decomposition decreases with increasing temperatures and decreasing pressures. In line with rather larger energy barriers, pressures larger than 10−6 bar are in general sufficient for ensuring a saturation of the computed unimolecular kinetic rate constants compared with the high-pressure limit (TST) of the RRKM unimolecular rate constants. The bonding evolution theory indicated that thermal decomposition of 2,3-epoxy-2,3-dimethylbutane into the 2,3-dimethylbut-3-en-2-ol takes place along three differentiated successive structural stability domains after passing the reactant from the associated transition state.


 Please wait while we load your content...
Please wait while we load your content...