Stereoselective glycoconjugation of steroids with selenocarbohydrates†
Abstract
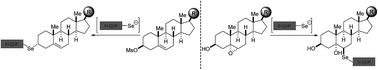
A methodology that brings together sugar and steroid scaffolds linked by a selenium atom is discussed in this work. A series of 6β and 3α glycoconjugated steroids were achieved by stereoselective nucleophilic substitution of cholesterol, pregnenolone, stigmasterol and sitosterol with different seleno-pyranosides and furanosides.

 Please wait while we load your content...
Please wait while we load your content...