Bromine substituted aminonaphthoquinones: synthesis, characterization, DFT and metal ion binding studies†
Abstract
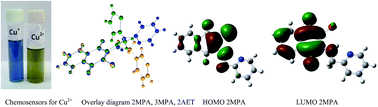
Bromine substituted aminonaphthoquinone ligands viz. 2-(2′-aminomethylpyridyl)-3-bromo-naphthalene-1,4-dione; (2MPA), 2-(3′-aminomethylpyridyl)-3-bromo-naphthalene-1,4-dione; (3MPA), 2-(2′-aminoethylpyridyl)-3-bromo-naphthalene-1,4-dione; (2EPA) and 2-(2′-aminomethylthiophenyl)-3-bromo-naphthalene-1,4-dione (2AMT) and 2-(2′-aminoethylthiophenyl)-3-bromo-naphthalene-1,4-dione (2AET) have been synthesized and characterized by single-crystal X-ray diffraction experiments in conjunction with long range corrected density functional theory. The heterocyclic amines on the naphthoquinone ring render diverse crystal structures. It has been shown that bromine substitution influences the planarity and mutual orientation of naphthoquinone and heterocycles in aminonaphthoquinone ligands. The 2MPA, 3MPA, 2EPA, 2AET aminonaphthoquinone ligands crystallize in the monoclinic space group whereas 2AMT led to the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Furthermore, molecular packing of 2MPA and 2EPA revealed dimeric structures while 3MPA and 2AMT are rendered with ‘stair-case’ arrangements of molecules. 2AET, when viewed down c-axis, showed a ‘butterfly like’ arrangement. A broad charge transfer band (400–600 nm) was observed in the UV-visible spectra of these ligands. Besides 2MPA and 2EPA exhibit remarkable selectivity toward Cu2+ ions, accompanied by a color change from orange to blue in methanol and methanol–water mixture.
. Furthermore, molecular packing of 2MPA and 2EPA revealed dimeric structures while 3MPA and 2AMT are rendered with ‘stair-case’ arrangements of molecules. 2AET, when viewed down c-axis, showed a ‘butterfly like’ arrangement. A broad charge transfer band (400–600 nm) was observed in the UV-visible spectra of these ligands. Besides 2MPA and 2EPA exhibit remarkable selectivity toward Cu2+ ions, accompanied by a color change from orange to blue in methanol and methanol–water mixture.

 Please wait while we load your content...
Please wait while we load your content...