Stereoselective innovative synthesis and biological evaluation of new real carba analogues of minimal epitope Manα(1,2)Man as DC-SIGN inhibitors†
Abstract
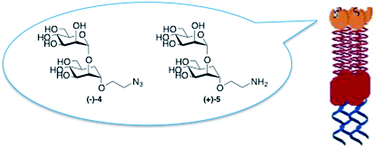
Antagonists of the C-type lectin DC-SIGN are promising therapeutic agents against viruses and bacteria. The development of glycomimetic ligands for DC-SIGN has so far proved to be challenging, since this membrane-protein presents four carbohydrate-binding domains (CRD) that specifically recognize mannose and fucose. In the recent past, we were able to develop inhibitors mimicking the minimal natural epitope Manα(1,2)Man using a mannoside with conformationally restricted dimethyl cycloexandicarboxylate-based aglycons designed to exploit the high enzymatic stability and to generate multivalent or solid supported systems as potent lectin ligands. Herein we describe the innovative synthesis of a different class of pseudodisaccharides, mimics of the natural Manα(1,2)Man moiety, characterized by the presence of a real D-carbamannose unit instead of a simpler mimic structure. Their chemical synthesis and biological activity using an SPR inhibition assay are reported. These pseudodisaccharides display inhibition values similar to those of the natural disaccharide Manα(1,2)Man, with a good affinity for DC-SIGN and can be considered as possible candidates for further structural modifications towards improved inhibitors.


 Please wait while we load your content...
Please wait while we load your content...