Galvanic replacement mediated synthesis of rGO–Mn3O4–Pt nanocomposites for the oxygen reduction reaction†
Abstract
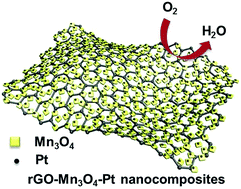
Pt-based nanomaterials are considered to be the most efficient electrocatalysts in various energy devices. Here we demonstrate a galvanic replacement mediated synthesis of hybrid rGO–Mn3O4–Pt nanocomposites as efficient electrocatalysts for the oxygen reduction reaction (ORR) in alkaline solutions. rGO–Mn3O4–Pt nanocomposites were obtained through galvanic replacement between Mn3O4 and Pt4+ ions, where the 8–10 nm Mn3O4 was homogeneously fabricated on the rGO surface. It was found that the distribution of Pt nanoparticles could be effectively modulated by the amount of Pt4+ ions. The as-prepared rGO–Mn3O4–Pt-1, with 2–3 nm Pt nanoparticles uniformly dispersed on the rGO–Mn3O4 support and 10 wt% Pt loading, even displays superior ORR catalytic activity to the commercial 20% Pt/C material. We believe the as-prepared rGO–Mn3O4–Pt nanocomposites are appealing for energy devices due to their excellent electrocatalytic performance and the decreased use of precious Pt.

 Please wait while we load your content...
Please wait while we load your content...