Preferred formation of the carboxylic acid–pyridine heterosynthon in 2-anilinonicotinic acids†
Abstract
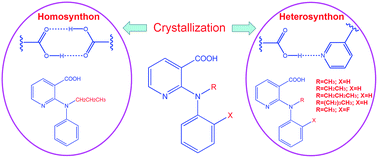
The carboxylic acid–carboxylic acid homosynthon and carboxylic acid–pyridine heterosynthon are two competing supramolecular synthons in 2-anilinonicotinic acids that possess both carboxylic acid and pyridine functionalities. Previously we demonstrated that carboxylic acid–pyridine heterosynthons can be selectively formed in crystals by chemically introducing bulky functional groups to the aniline ring of the molecules. In this study we show that with the same philosophy, but a different strategy, i.e., adding substituent groups to the nitrogen bridging the two aromatic rings, we can also achieve the preferential formation of the carboxylic acid–pyridine heterosynthon over the carboxylic acid–carboxylic acid homosynthon. This is a new case of how molecular conformation can affect intermolecular interactions and consequent crystal packing.

 Please wait while we load your content...
Please wait while we load your content...