Strained alkyne substituted near infrared BF2 azadipyrromethene fluorochrome†
Abstract
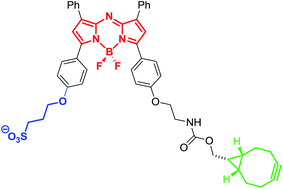
The contribution of imaging with near infrared (NIR) fluorophores to the elucidation of complex biological processes continues to rapidly expand. New tools for mild selective bioconjugation are strongly desirable for the construction of NIR labelled biomolecules. This work presents the synthesis and evaluation of a strained alkyne substituted NIR BF2-azadipyrromethene (NIR-AZA) which can conjugate in water with azido-homoalanine and cRGD peptide within 30 min at rt. Imaging analysis of the NIR-AZA–cRGD conjugate in vitro and in vivo showed efficient cellular uptake and good in vivo tumor targeting properties, illustrating the potential for translation to clinical imaging.

 Please wait while we load your content...
Please wait while we load your content...