Interactions of the N-terminal domain of human islet amyloid polypeptide with lipid membranes: the effect of cholesterol†
Abstract
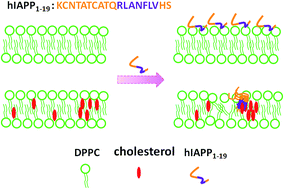
The 1–19 region of human islet amyloid polypeptide (hIAPP1–19) is a dominating factor causing the interaction between hIAPP and membrane. It contains a short sequence RLANFLV that fulfils the amino-acid arrangement of the inversed cholesterol recognition amino-acid consensus (CARC) and may mediate a direct contact of hIAPP with cholesterol. In this study, we focused on the interaction of hIAPP1–19 with the lipid membrane composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and cholesterol, and examined the role of the CARC motif in the peptide–membrane interaction. Using differential scanning calorimetry, 31P-NMR spectroscopy, 1H-NMR titration measurement and dye leakage assay, we demonstrated that hIAPP1–19 interacts with DPPC vesicles more strongly in the presence of cholesterol than it does in the absence of cholesterol. The peptide–membrane interaction promotes the domain segregation of the raft-containing membrane. The peptide is more disruptive to the cholesterol-containing membrane than it is to the cholesterol-depleted membrane. The substitution of the residue Phe at position 15 of hIAPP1–19 by Leu leads to a distinct decrease in the peptide–membrane interaction in the presence of cholesterol, but the effect of the residue substitution on the peptide–membrane interaction is very small in the absence of cholesterol. The circular dichroism data indicated that a conversion of the structure from a random coil to an α-helix is induced by cholesterol for both peptides and the structural conversion is more Chol-dependent for the wild-type peptide than the F15L variant. Our findings suggest that cholesterol could facilitate the insertion and aggregation of the N-terminal domain of hIAPP in the membrane, and the phenylalanine in the CARC motif could be involved in the interaction of the N-terminal domain with Chol.

 Please wait while we load your content...
Please wait while we load your content...