Copper(i)-mediated synthesis of β-hydroxysulfones from styrenes and sulfonylhydrazides: an electrochemical mechanistic study†
Abstract
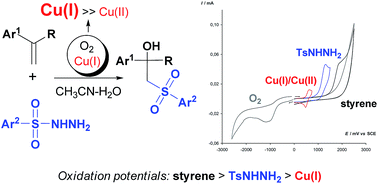
Copper(I) halides were used as mediators in the synthesis of β-hydroxysulfones via the oxysulfonylation of styrenes using sulfonylhydrazides. The feature of the developed process lies in the combination of a copper(I) salt with oxygen—the stoichiometric oxidant. Copper(II) species are responsible for the oxidation of sulfonylhydrazides, they are generated in small amounts in the O2/Cu(I)/Cu(II) redox system, which is formed during the reaction. The combination of these three components enables one to obtain in the case of α-methylstyrenes only β-hydroxysulfones and in the case of α-unsubstituted styrenes, β-hydroxysulfones as the main products and β-ketosulfones as the by-products. With good yields β-hydroxysulfones were prepared by reduction of the reaction mixture containing both products β-hydroxysulfones and β-ketosulfones with NaBH4. An electrochemical study revealed that the Cu(I)/Cu(II) pair can serve as an effective mediator of β-hydroxysulfones formation via redox processes.


 Please wait while we load your content...
Please wait while we load your content...