Improved corrosion resistance by forming multilayers over a copper surface by electrodeposition followed by a novel sol–gel coating method
Abstract
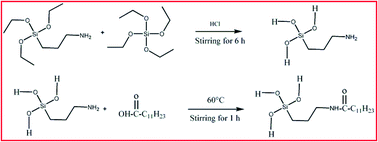
Facile nickel coating on a copper surface was successfully fabricated via an electrodeposition method from nickel ammonium sulfate and then surface modification was done by subsequent immersion into the sol–gel mixture of 3-aminopropyltriethoxysilane (APTES), tetraethoxysilane (TEOS) and lauric acid (LA). The surface morphology, chemical composition and wettability were characterized by means of scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier transform infrared (FT-IR) spectroscopy and water contact angle (WCA) measurements. It was found that the Cu–Ni–Hy–LA multilayered sol–gel coated surface was hydrophobic with a contact angle 107.3°. The corrosion resistance of these coatings was evaluated by electrochemical impedance studies (EIS) and potentiodynamic polarization (PP) measurements in a 3.5% NaCl medium. The Cu–Ni–Hy–LA multilayered sol–gel coating showed enhanced corrosion resistance compared to the Cu–Ni and Cu–Ni–Hy surface. The improved corrosion resistance is mainly due to the formation of a densely packed sol–gel coating over an electrodeposited copper surface.

 Please wait while we load your content...
Please wait while we load your content...