Nitroxide-mediated polymerization of methyl methacrylate by 4,4′-dimethoxydiphenyl-based alkoxyamine†
Abstract
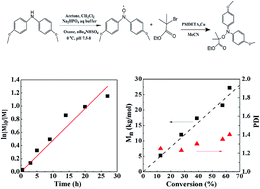
Nitroxide-mediated polymerization of methyl methacrylate (MMA) was carried out using 4,4′-dimethoxydiphenyl nitroxide-based alkoxyamine as a mediator. This alkoxyamine can be easily prepared and is able to control the nitroxide-mediated polymerization of MMA up to a conversion of 65%. A linear increase of the number-average molecular weight with conversion was observed and polymers with narrow molecular weight distributions (PDI = 1.2–1.4) were obtained. The living character was also confirmed by chain extension from the prepared poly(methyl methacrylate) macroinitiator with MMA or styrene. ESR (electron spin resonance) results indicate that the living chain fraction is more than 85%. Additionally, the MMA polymerization cannot be well controlled by the diphenylnitroxide-based alkoxyamine because of the unwanted cross combination reaction between two nitroxides; the prepared polymer shows a bimodal and broad molecular weight distribution.


 Please wait while we load your content...
Please wait while we load your content...