Fuchsine biosorption using Asplenium nidus biosorbent-a mechanism using kinetic and isotherm data
Abstract
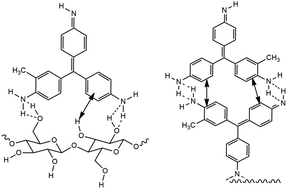
Textile dye contamination of waterways is a major environmental and health issue related to small and medium size enterprises in developing countries. Conventional decontamination techniques are expensive for these enterprises. Biosorption is cost effective, simple and an efficient method for decontamination. An understanding of the adsorption mechanism under optimum reaction conditions would enable the efficient utilization of the biosorbent. We determined the adsorption behaviour of a biosorbent prepared from the ornamental fern A. nidus and fuchsine dye under different experimental parameters. Kinetic data were fitted to adsorption kinetic models and adsorption diffusion models. Isotherm data were fitted to two-parameter and three-parameter isotherm models. This paper postulates a mechanism for the adsorption of fuchsine dye onto the biosorbent using kinetic, isotherm and thermodynamic data. The biosorbent adsorbed 88% of fuchsine after 150 min, under the experimental conditions. The adsorption percentage increased when the biomass dose was increased from 0.1 g to 0.2 g and remained the same thereafter. Kinetic data showed that the pseudo second order kinetic model is more applicable and both intraparticle diffusion and liquid film diffusion control the rate of the adsorption process. Isotherm studies showed that the Langmuir–Freundlich isotherm model explains the adsorption process well with a maximum adsorption capacity of 12.95 mg g−1 of dry biosorbent. Thermodynamic parameters suggest that the adsorption is a spontaneous exothermic process with an enthalpy change of −59.26 kJ mol−1 and entropy change of −0.09 kJ mol−1 K−1. It can be concluded that the adsorption is governed by diffusion through the liquid film and within the biosorbent particle surface forming covalent and hydrogen bonding interactions between fuchsine molecules and functional groups of the adsorbent and π–π electron interactions between phenyl rings of the dye molecule.

 Please wait while we load your content...
Please wait while we load your content...