Additive effect of alkaline earth metals on ammonia decomposition reaction over Ni/Y2O3 catalysts†
Abstract
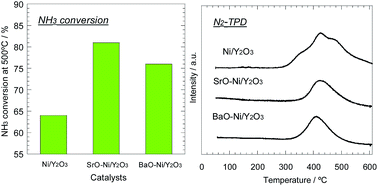
Recently, ammonia has attracted much attention as a promising hydrogen carrier due to various advantages for on-site hydrogen generation. In this study, Ni/Y2O3 catalysts modified by alkaline earth metals were prepared and their catalytic activity for ammonia decomposition was examined. The addition of a small amount of Sr or Ba species remarkably improved the performance of Ni/Y2O3, while the Mg- and Ca-modification were not effective. The XRD analysis elucidated that the composite oxides consisting of alkaline earth metals and nickel were formed in the as-calcined Sr- and Ba-modified Ni/Y2O3 catalysts, and were decomposed by the reduction treatment. This suggested that the Sr and Ba components were located in the vicinity of Ni particles, resulting in the strong interaction with the Ni species. The NH3-temperature programmed surface reaction measurement revealed that the desorption of nitrogen atoms strongly-adsorbed on the Ni surface has terminated at lower temperatures over the Sr- and Ba-modified catalysts than over the others. This characteristic desorption behavior would be mainly attributed to the enhancement of the electron density in the Ni metal by the strong basic property of Sr and Ba components and the strong Ni–Sr and Ni–Ba interaction. Considering the nitrogen desorption step was kinetically significant, this change in the electronic state of the Ni surface should be responsible for the promotion effect of the Sr- and Ba-modification.


 Please wait while we load your content...
Please wait while we load your content...