Mononuclear copper(ii) complexes of an arylhydrazone of 1H-indene-1,3(2H)-dione as catalysts for the oxidation of 1-phenylethanol in ionic liquid medium†
Abstract
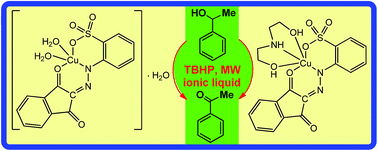
Sodium 2-(2-(1,3-dioxo-1H-inden-2(3H)-ylidene)hydrazinyl)benzenesulfonate (NaHL) was synthesized by azocoupling of 2-sulfobenzenediazonium chloride with 1H-indene-1,3(2H)-dione in the presence of sodium hydroxide. The reaction of copper(II) nitrate hydrate with NaHL in the absence or presence of diethanolamine (H3L1) leads to the new complexes [CuL(H2O)2]·H2O (1) and [CuL(H3L1)] (2), respectively. The compounds are water soluble and were characterized by elemental analysis, ESI-MS, IR spectroscopy, single crystal X-ray diffraction analysis (for 1 and 2) and, for NaHL, by 1H and 13C NMR spectroscopies. In both 1 and 2, L2− works as a chelating ligand, and a crucial synthetic and structural role is played by H2O and H3L1. The catalytic activity of 1 and 2 was successfully tested in the oxidation of 1-phenylethanol using microwave irradiation and two media, a solvent-free and a metal containing ionic liquid (IL), methyltrioctylammonium tetrachloroferrate(III) ([N1,8,8,8][FeCl4]). The possibility of recycling without loss of activity (for 2) and of reusing the IL is demonstrated, as well as the dual role played by this IL as both a solvent and promoter of the alcohol oxidation. To our knowledge it is the first time that a copper(II) complex derived from arylhydrazones of 1H-indene-1,3(2H)-dione has been tested in alcohol oxidation catalysis.


 Please wait while we load your content...
Please wait while we load your content...