Rh-catalyzed direct synthesis of 2,2′-dihydroxybenzophenones and xanthones†
Abstract
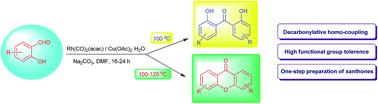
An efficient rhodium-catalyzed direct synthesis of 2,2′-dihydroxybenzophenones and xanthones was developed from functionalized salicylaldehydes. This approach provides an easy access to various functionalized 2,2′-dihydroxybenzophenone and xanthone core skeletons. This study also revealed the crucial role of the hydroxy group in the reductive homo-coupling process to generate 2,2′-dihydroxybenzophenones. Overall the outcome of the reaction course was also found to be influenced by the electronics of the substituent groups in salicylaldehydes.

 Please wait while we load your content...
Please wait while we load your content...